Pages That Need Review
Research Notes III, 1950
21
11/17/50
24.043 gms of triply distilled water was added to 4.225 gms MnSO₄·H₂O to form 25 cc. 1. Molar MnSO₄ solution
Percent H₂O in this solution = 97.9%
1/19/50
4.256 g. 99.5% D₂O was added to 0.507 g. MnSO₄·H₂O to form a 4 cc. solution.
Molarity of MnSO₄ = 3/4 Volume of D₂O - 3.830 cc Molarity of H = 1.28
25
11/23/50
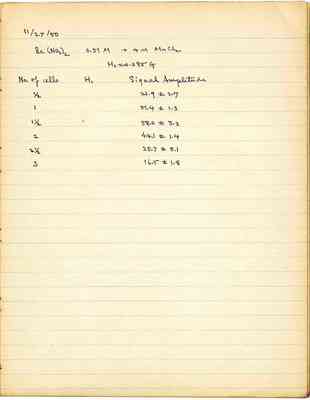
Proton resonances from the following two samples were compared under identical conditions:
Sample volume = 4 cc Molarity of Mn++ = 0.75 Gain = 60 Hs = 0.086 G H₁ = 1 1/2 cell ~ 0.089 G Rate = D H₀ ~ 1500 G
(a) 4.256 g 99.5 % D₂O was added to 0.507 g. MnSO₄·H₂O to form 4 cc solution. It contains H to 1.28 M (b) 4 cc. of 0.75 M Mn++ in H₂O
Size of signal from sample (a) = 65.1
" " " " " (b) = 13.5 (attenuated to a factor 1/200)
Conclusion: [illegible] If attentuation factor is correct, % purity of D₂O is 98.2%
27
12/1/50
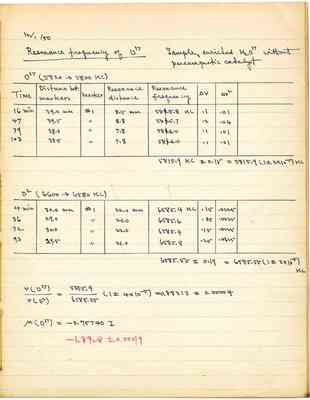
Resonance frequency of O17
Sample: enriched H₂O17 without paramagnetic catalyst
O17 (5820 --> 5800 KC)
| Time | Distance bet. markers | marker | Resonance distance | Resonance frequency | Δν | Δν² |
|---|---|---|---|---|---|---|
| 16 min | 39.0 mm | #1 | 8.5 mm | 58 & 5.8 KC | .1 | .01 |
| 47 | 39.5 | " | 8.8 | 58 & 5.7 | .2 | .04 |
| 79 | 38.0 | " | 7.8 | 58 & 6.0 | .1 | .01 |
| 103 | 38.5 | " | 7.8 | 58 & 6.0 | .1 | .01 |
| 4 min | 30.0 mm | # 1 | 22.0 mm | 6585.4 KC | .15 | .0225 |
|---|---|---|---|---|---|---|
| 36 | 29.0 | " | 21.0 | 6585.6 | .05 | .0025 |
| 72 | 30.0 | " | 22.0 | 6585.4 | .15 | .0225 |
| 93 | 29.5 | " | 21.0 | 6585.8 | .25 | .0625 |
29
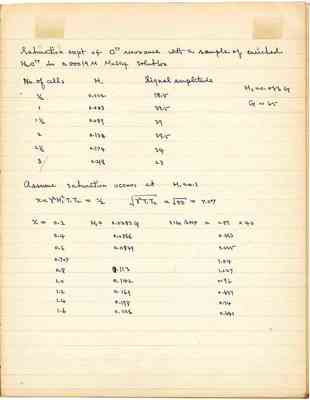
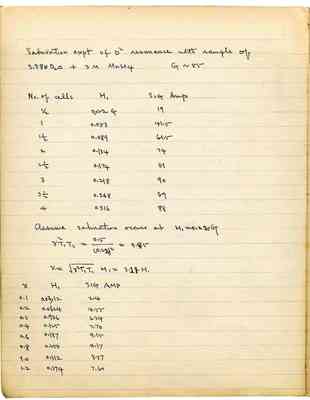
Saturation expt of O17 resonance with a sample of enriched H2O17 in 0.00019 M MnSO4 solution
| No. of cells | H₁ | Signal amplitude |
|---|---|---|
| 1/2 | 0.022 | 18.5 |
| 1 | 0.053 | 33.5 |
| 1 1/2 | 0.089 | 39 |
| 2 | 0.134 | 39.5 |
| 2 1/2 | 0.174 | 34 |
| 3 | 0.218 | 23 |
Assume saturation occurs at H₁ = 0.1
| x | H₁ | SIG AMP |
|---|---|---|
| 0.2 | 0.0283 G | 0.51 x 40 |
| 0.4 | 0.0566 | 0.864 |
| 0.6 | 0.0849 | 1.025 |
| 0.707 | - | 1.04 |
| 0.8 | 0.113 | 1.027 |
| 1.0 | 0.142 | 0.96 |
| 1.2 | 0.169 | 0.851 |
| 1.4 | 0.198 | 0.74 |
| 1.6 | 0.226 | 0.641 |
30
Saturation expt of D² resonance with sample of 3.38 N D₂O + 3 M MnSO₄
G ~ 55
| No. of cells | H₁ | Sig Amp |
|---|---|---|
| 1/2 | 0.022 G | 19 |
| 1 | 0.053 | 41.5 |
| 1 1 /2 | 0.089 | 61.5 |
| 2 | 0.134 | 74 |
| 2 1/2 | 0.174 | 81 |
| 3 | 0.218 | 90 |
| 3 1/2 | 0.268 | 89 |
| 4 | 0.316 | 88 |
| x | H₁ | SIG AMP |
|---|---|---|
| 0.1 | 0.0312 | 214 |
| 0.2 | 0.0624 | 4.55 |
| 0.3 | 0.936 | 6.34 |
| 0.4 | 0.125 | 7.70 |
| 0.6 | 0.187 | 9.15 |
| 0.8 | 0.250 | 9.17 |
| 1.0 | 0.312 | 8.57 |
| 1.2 | 0.374 | 7.60 |
31
12/10/50
The siqnal amplitudes & width of O17 & D2 resonances were compared at the same magnetic field
O17 Height = 41.7 ± 2.6 , Width = 40.0 ± 2.4 enriched H2O17 +0.00019M MnSO4
D2 1.20N D2O Height = 82 ± 4 Width = 66 ± 4 +3M MnSO4
a ≡ amplitude
I (I+1) n γ3 / ΔH2 ------------------------------------------------------------ ID(ID+1) nD γ3D / ΔH2D
= a ----- aD
I(I+1) = ID(ID+1)(nD / n)(γD / γ)3 {aΔH2 / aDΔH2}
= 2x 1.70/0.1 x 1.45 x 42x402/82x662 (1+14.5%) x 0.96 correction factor for difference in rates
=8.90 (1+14.5%)
I = 2.52 ± 0.[?] % abundance of O17 = 0.18 Molarity of O17 = 55.55 x 0.18 = 0.1