Pages That Need Review
Research Notes I, 1949
79
Dec. 9 1949 Observations of the resonance of N14 were made with the following chemical compounds: K3 Fe(CN)6 , K4Fe(CN)6, K3 Co(CN)6. The results were N14 - resonance Compounds occures at same place K3Fe(CN)6 Fe has no 213 uB signal yes K4Fe(CN)6 yes K3Co(CN)6 This indicates that not only the complex K4Fe(CN)6 K3Co(CN)6 are diamagnetic, the Fe [illegible] Co in the compounds are themselves diamagnetic
83
a35/a37 = 1.202723 ~ 9400 grams Cl35 (3920->3910) Time Dist. bet Marker Resonance [illegible] v [illegible]v2 Marker Dist. freq 22 min 113.5 mm (15 KC) #2 102.5 mm 3911.3 KC +0.15 0.0225 72 76.0 #1 68.0mm 3911.2 0.05 0.0025 109 68.5 #2 60.0 3911.1 -0.05 0.0025 146 68.3 #2 60.5mm 3911.0 -0.15 0.0225 mean=3911.15 3 0.0500 .0.170 v(cl35)=(3911.125+-0.13)KC =3911.15(1+-3x10 5) v(cl35)/v(cl37)=1.20138(1+-8x10-5) =1.20138+-0.00008 935/937=1.202723 Cl37(3260->3250) Time Dist bet Marker Marker Resonauce Dist Resonauce beg [?][?] 29min 75.0mm #2 30.0mm 3255.9KC +0.35 0.1225 84 75.4" #2 31.5 3255.7 +0.15 0.0225 121 79.0" #2 35.0 3255.5 -0.05 0.0025 159 78.5" #2 35.0 3255.5 -0.05 0.0025 mean=3255.55 3 0.1500 0.0500 v(cl37)=(3255.55+-0.22)KC =3255.55(1+-7x10-5) 1.2013791 0.684380
85
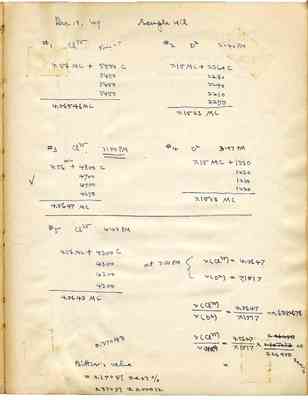
Dec 19, '49 Sample HCl #1 Cl35 Time=? #2 D2 3:40PM 4.56MC +5500 C 7.15MC+2360C 5450 2280 5450 2240 5450 2210 5450 2250 4.56546 MC 7.1523MC #3 Cl35 3:50 PM #4 D2 3:57 PM 4.56MC +4800 C 7.15MC +1330 4700 1230 4700 1280 4650 1220 4.5647 MC 7.1513 MC #5 Cl35 4:03 PM 4.56 MC+4300C at 3:50PM v(cl35)=4.5647 4300 v(D2)=7.1517 4300 4300 4.5643MC v(Cl35)/v(D2)=4.5647/7.1517=0.6382678 v(Cl35)/v(Na23)=4.5647/7.1517x[?]/0.26450+-0.01% 0.37043 Bitter's value=0.37051+-0.03% 0.37051+-0.00012
90
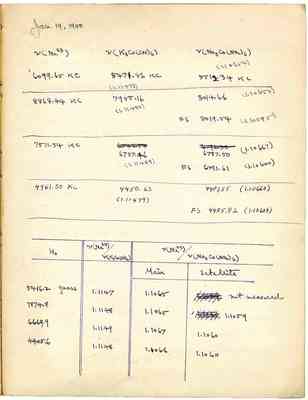
Jan. 19, 1950 v(Na23) v(K3Co(CN)6) v(Na3Co(NO2)6) (1.10654) 6099.65 KC 5471.86 KC 5512.34 KC (1.11473) 8868.44KC 7955.16 8014.66(1.10653) (1.11480) FS 8019.54(1.10585) 7511.54KC 6737.46 6787.50 (1.10667) (1.11489) FS 6791.61 (1.10600) 4961.50KC 4450.63 4483.55(1.10660) (1.11479) FS 4485.82(1.10604) Ho v(Na23)/v(K3Co(CN06) v(Na23)/v(Na3Co(no2)6) 5416.2 gauss 1.1147 1.1065 not measured 7874.8 1.1148 1.1065 1.1059 6669.9 1.1149 1.1067 1.1060 4405.6 1.1148 1.1066 1.1060
92
The function at its poles inside the unit circle around the origin.
The zeros of are the zeros of the two equations
If the zeros of (1) are z1 & z2, the zeros of (2) are the complex conjugates . If z1 lies inside the unit circle, so does . Therefore z1 & are the only two [principle?] poles inside the circle. Thus
The residue of f(z) at
The residue of f(z) at
98
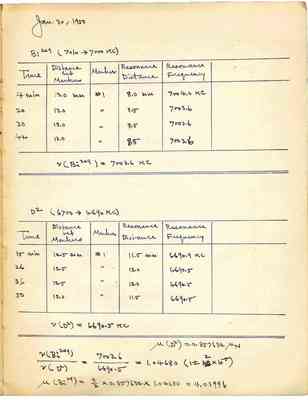
Jan. 30, 1950 Bi209 (7010-7000KC) Time Distance bet Markers Marker Resonance distance Resonance frequency 4 min 13.0 mm #1 8.0 mm 7004.0KC 20 13.0 " 8.5 7003.6 30 13.0 " 8.5 7003.6 42 13.0" 8.5 7003.6 v(Bi209)=7003.6KC D2(6700->6690KC) Time Distance bet Markers Markers Resonance Distance Resonance Frequency 15 min 12.5 mm #1 11.5 mm 6690.9KC 26 12.5 " 12.0 6690.5 36 12.5 " 12.0 6690.5 50 12.0 " 11.5 6690.5 v(D2)=6690.5 KC v(Bi209)/v(D2)=7003.6/6690.5=1.04680(1+-2x10-5) u(Bi29)=9/2x0.857632x1.04680=4.03996
100
Feb. 13, '50
N14 occurs at 3.34 MC at H0 = 10600 G in K3Co(CN)6. Search for N14 in Na3Co(NO2)6 in freq range 3.445 MC - 3.26 MC is negative
As to the structure of NaSbF6 refer to Nils Schrewelius, Röntgenuntersuchung der Verbindungen NaSb(OH)6, NaSbF6, NaSbO3 und gleichartiger Stoffe. Zeitschrift für anorganische und allgemeine Chemie Bd 238, 248 (1938)
NaSbF6 is cubic a = 8.18 Å The structure is probably the space group 4 Na in 4(b) , 4 Sb in 4(a), 24 F in 24(d) ; x = -0.05, y = -0.05 , z = 0.225
within the SbF6- octahedron 2.67, 2.78 and from octahedron [?] octahedron 2.72 Å
108
HSbCl6 was prepared by treating chemically pure SbCl₅ with 10% (~3 N) hydrochloric acid, both of the reagents were precooled to the temp of ice water. [left margin]a fuming liquid[/margin]
HSbCl6 is unstable at room temperatures Signal amplitude was reduced to about 20% after standing for 24 hours.
Suggestion: mean life time of the compound can be measured with the spectrometer.