Pages That Need Review
Research Notes I, 1949
59
Sept. 29 49 Mo-sample m.w. = 238.14 (A) 13.58 guess of K2M0o4 in 12 ml of H2O Mo #1 4.75 M of Mo 2 o M of Co(NO3)2 Mo #2 M of Mo 2 M of Co(NO3)2 (B) 17.46 gm of K2MoO4 m 18 ml of H20 Mo #3 407 M of Mo 2 M of Co (NO3)2 Mo #4 407 M of Mo 2 M of Co (NO3)2 Mo #5 407 M of Mo M of Co (NO3)2
62
Mn#1 0.18M KMnO4 Resonance at 6.87 MC 7.21 MC 0.8 M Co(NO3)2 Ho = 6600 grams Mn#2 0.151 gms KMn04 in 5c.c, soln 3c.c. of Co(No3)2(2 molar) in 5c.c. soln Molarity of Mn=0.151/5/0.158=0.191 Molarity of Co++=3/5x2=1.2 v(Na23)=8845.4+-0.2KC v(Mn55)=8290.0+-0.2KC Dec14, '49 checked by using GR No.271 v(Mn55)/v(Na23)=6.8411/7.3001=0.932124 v(Mn55)/v(Na23)=8290.0/8845.4(1+-3x10-5)=0.937210 M(Na23)/3=0.738875MN M(Mn55)=3.46241Mn(1+-0.01270) =+(3.4624+-0.0004)MN M(Na23)/M(H1)=0.26450+-0.01% M(H1)=2.79348(1+0.00018)
69
N#2 19 c.c. soln contains 12 grams of NH4NO3 2 10 c.c. of 3.85 M soln of MnCl2 Molarity of N = 16M Molarity of Mn [illegible] = 2M N#3 18 c.c. soln contains 12.5 grams NH4NO3 10 c.c. of 3.8512 M soln of MnCl2 Molarity of N = 17.4 M Mn [illegible] = 1 M N#4 12.5 grams NH4NO3 in 18 cc H20 ; 0.2 M MnNO3 N A 1.948 g Cr(NO3)3 9 H2O 1.948 x 42/400.2= 0.204 g B 1.832 g NH4NO3 1.832x28/80 = 0.64 g C 2.240 g NH3 2.240x14/17 = 1.84 g Ration of A to (B+C) = 0.204/2.48 = 0.082 Total nitrogen content in NH3 = 2.18 g. Total nitrogen content = 2.64 g. Ratio = 2.18/2.64 = 0.825 0.825/15 = 0.055
70
Fine structure Expts
I. Fine structure of N14 resonance line was first noticed in sample containing liquid NH3 + NH4NO3 + Cr(NO3)2 · 9H2O
II. Demonstrated it was not Cr resonance by using samples
(1) Cr #1 12 M CrO3 in H2O (2) Cr #2 5M CrO2 in H2O + 1 M MnSO4 (3) Cr #3 1.25 M Cr(NO3)3
III. Crude expts show that Cu(NO3)2, Cr(NO3)2 and NH4NO3 give resonances nearer that freq of fine structure than nearer the big resonance
[diagram] [up arrow] Freq down
IV. Sample NH4NO3 + MnNO3 showed F.S. too !!!
75
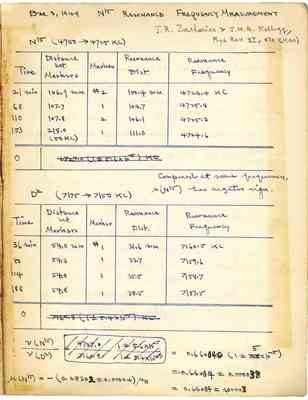
Dec 9,1949 N15 RESONANCE FREQUENCY MEASUREMENT J. R .Zacharias &J.M.B.Kellogg,Plus Rev. 57,570(1940) N15(4750->4725KC) Time Distance bet Markers Marker Resonance Dist Resonance Frequency 21Min 106.9mm #2 100.4mm 4726.4KC 68 107.7 1 104.7 4725.8 110 107.8 2 106.1 4725.3 153 218.0 1 111.0 4724.6 (50KC) Compared at same frequency, M(N15)has negative sign. D2 (7175->7150KC) Time Distance bet. Markers Marker Resonance Frequency 36min 54.0mm #1 31.6mm 7160.5KC 80 54.3 1 33.7 7159.6 124 54.0 1 35.5 7158.7 188 54.8 1 38.5 7157.5 0 V(N15)/V(D2)=4727.0/7160.8 1+-5.6x10-5/1+-5.4x10-5=0.660050(1+-5x10-5) =0.66004+-000033 =0.66004+-0.00003 M(N15)=-(0.283032+-0.00004)MN
77
H. Kopfermann [und] E. Rasmussen, Zeit f. Phys., 98, 624 (1936).
They were pretty sure that the spin of V51 is 7/2.
Auf Grunde dieser Überlegungen kommt zur Sicherstellung des Vanadium kernmoments wegen ihrer besonders großen J-Werte vor allem die Linie λ = 4591 Å 3d^3 4s^2 4F 9/2 - 3d^3 4s 4p ^4 G 11/2) in Frage. Es komiten die ersten fünf Komponenten und die Gesamtbreite ein wande frei gemessen werden. Eine schwache vorgelageite Komponente existiert nicht. Das Intervall verhältnis der gewesen Komponenten entspricht sehr genau dem Verhältnis der F-Werte das oberen Zustandes für I=7/2. Die daraus extrapotierte Gesamt breite stimmt mit der gemessen innenhalb der Fehlergrenzen überein. Der Wert I=9/20 für den zwar die Intervall regal auch noch genähert -- allerdings wesentlich schlecter als für I=7/2 -- erfüllt ist, scheidet mit Sicherheit aus, da ihm wegen der dann zu erwartenden zehn tashen Aufspaltung der Terme eine Gesamtbreite der Linie entsprechen würde, die um 0.05 cm-1 großer sein müßte, als die beobachtete Breite.
78
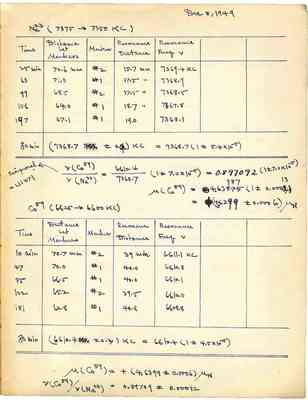
Dec 8, 1949 Na23 (7375 -> 7350 KC) Time Distance Markers Resonance Resonance bet Marker Distance Freq v 25 min 70.6 mm #2 15.7 mm 7369.4 KC 63 71.0 #1 17.5 " 7368.9 99 68.5 #2 17.5" 7368.5 156 64.0 #1 18.7" 7367.8 197 67.1 #1 19.0 7368.1 80 min (7368.7+-0.4)KC =7368.7(1+-5.4x10-5) Reciprocal=1.11471 v(Co59)/v(Na23)=6610.4/7368.7(1+-7.0x10-5)=0.897092(1+-7.0x10-5) M(Co59)+4.63987(1+-0.00013) =(4.6399+-0.0006)MN Co59(6625->6600KC) Time Distance bet Markers Marker Resonance Distance Resonance Freq v 10 min 70.7mm #2 39 min 6611.1 KC 47 70.0 #1 40.0 6610.8 85 66.5 #1 40.0 6610.1 122 65.2 #2 39.5 6610.0 181 62.8 #1 40.8 6608.8 80 min (6610.4+-0.3)KC=6610.4(1+-4.5x10-5) M(Co59)=+(4.6399+-0.0006)MN v(Co59)/v(Na23)=0.89709+-0.00012